A vast number of chemicals are registered for production and use around the world. But only a portion have been thoroughly evaluated for their toxicity due to time, cost, ethical concerns and regulatory limitations.
To safeguard public health, researchers at organizations such as the U.S. Environmental Protection Agency, U.S. Food and Drug Administration and European Chemicals Agency evaluate the safety of the potentially hazardous chemicals people are likely to come into substantial contact with. These include volatile organic compounds such as formaldehyde, air pollutants such as nitrogen dioxide, consumer chemicals such as bisphenol A, and herbicides such as atrazine. Recently, “forever chemicals” that persist in the environment, such as perfluorooctanesulfonic acid (PFOS) and perfluorobutane sulfonic acid (PFBS), have been the focus of human toxicity assessments.
There are thousands of other chemicals used by industry that haven’t been thoroughly tested. To be efficient and cost-effective, these chemicals are prioritized for targeted testing. I am a toxicologist who studies how chemicals affect human health, particularly when they cause harmful effects. Better understanding the process of determining the toxicity of chemicals could help make them safer.
Chemical safety and toxicity testing
Historically, researchers have tested the safety and toxicity of chemicals by using biological assays, or bioassays. These tests involve exposing nonhuman animals – often rodents such as rats or mice – to a substance in controlled conditions to study its biological effects, including its potential harms.
Different types of studies are designed to analyze different effects from chemicals. These include immediate effects, effects from both short-term and long-term exposure, and reproductive or developmental effects. The key premise in using bioassays in animals is that researchers can use the results to help understand the chemical’s safety for people.
There are, however, significant limitations in using animals to conduct these studies.
First, it can be difficult to extrapolate results obtained from lab animals to humans. There are notable differences in anatomy, physiology, biochemistry and genetics between laboratory animals and people. In some cases, a chemical that is highly toxic to humans may be relatively harmless to other species. Moreover, even within a given species, there can be significant differences in how the body breaks down molecules, a process critical to determining a chemical’s toxicity.
It can also be costly to conduct research in animals. For example, a full battery of toxicology tests for a pesticide can cost between US$8 million and $16 million. Many of these studies take a long time to conduct, with some requiring up to two years.
There are ethical concerns, too, about using animals to test the toxicity of chemicals. Many governmental agencies and commercial entities have committed to efforts that replace, reduce or refine the use of animals in research and testing.
Researchers are developing a number of ways to replace animal testing in assessing chemical safety. Often called new approach methodologies, these methods aim to be both relevant to humans and scientifically clear. They also seek to be cost-effective, fast and broadly applicable.
In vitro tests
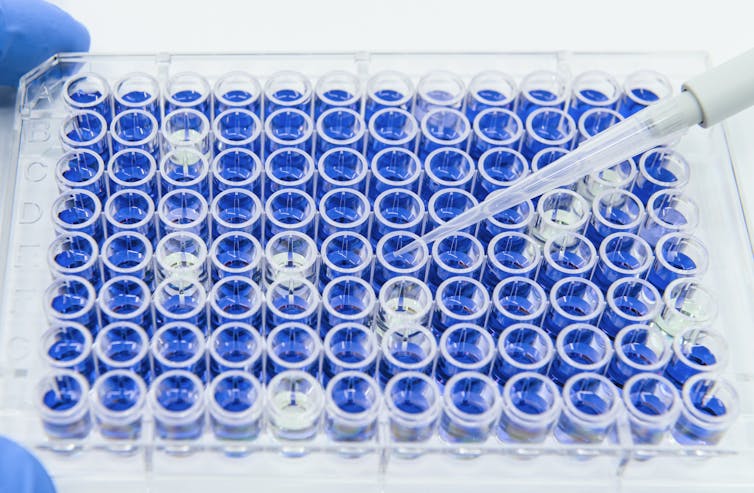
In vitro tests involve exposing biological materials such as human cells or microorganisms to different concentrations of a chemical of interest. These tests have several benefits, including easy control over experimental conditions, applicability to people, and the capacity to rapidly study many chemicals at once. The EPA’s ToxCast program uses data from in vitro tests to study thousands of chemicals.
In vitro tests are commonly used to study chemical toxicity.
unoL/iStock via Getty Images Plus
There are numerous types of in vitro tests, each studying a particular quality associated with toxicity. For example, cell viability assays measure the effect of a chemical on cell survival and growth. Genotoxicity assays evaluate whether a chemical can damage genetic material. And receptor binding assays assess whether chemicals can interact with specific proteins on cells and trigger harmful effects.
One type of in vitro cell model are organotypic cultures derived from actual tissues or organs. These models retain the structural and functional qualities of their original tissue.
Other cell models originate from cells that self-organize in three dimensions. Examples include organoids and bioprinted tissues that can be tailored to represent specific tissues, such as the liver, skin and heart.
Microphysiological systems, or organ-on-a-chip models, use miniature 3D cultures of cells from various organs – such as the liver, heart and lungs – to mimic how these organs would function in the body. With these models, researchers can assess how toxic a chemical is to multiple organs, how it is broken down in the body, and how it may cause disease. This technique offers the possibility to study the effects of chemicals on the body in a more realistic and holistic way than with organ-specific models.
Organs-on-chips are cell cultures that mimic entire organ systems.
In chemico assays
In chemico assays are laboratory tests or experiments that examine how chemicals interact with proteins, lipids or other cell components in a test tube or other synthetic platform. They are well suited for studies on the mechanisms underlying chemical interactions.
Compared to in vitro systems, in chemico assays can be faster and more cost-effective. They may also be ethically preferred since no live cells or tissues are used.
However, they may have limited biological relevance since they cannot account for how these chemicals would work in a living organism. They are also not suitable to study many aspects of chemical toxicity, such as how it affects the overall function of a cell or the body.
In silico methods
One important aspect of chemical toxicity is figuring out what doses of a chemical trigger an unwanted side effect, or its pharmacodynamics. Another is how much of the chemical gets to its target and over what period of time, or its pharmacokinetics.
When little to no experimental data is available about a chemical, researchers often rely on computer models, or in silico methods. Predicting a chemical’s dose response often relies on the idea that chemicals with similar structures will have similar biological effects. Thus, if a researcher has data on chemicals similar in structure to a chemical of interest, computational models could estimate how it will affect the body.
Physiologically based pharmacokinetic modeling mathematically divides the body into compartments.
Fuzzyrandom/Wikimedia Commons, CC BY-ND
Scientists often use what are called physiologically based pharmacokinetic models to predict how a drug travels through the body. This approach mathematically divides the body into compartments – such as the liver, kidney or blood – and simulates how the chemical moves between them based on its properties and the physiology of the body. Other in silico approaches, such as virtual tissue models and quantitative adverse outcome pathways, provide additional information on how chemicals cause adverse health effects.
In silico methods offer many advantages over traditional methods. They are faster and more efficient, and researchers can tailor virtual tests to more precisely simulate scenarios that would otherwise be infeasible to conduct. In silico methods are also easily replicable across labs and can help fill data gaps.
However, in silico methods also have several drawbacks. These include lower accuracy with faulty models, the need for experimental data to develop models, and the lack of standards to evaluate whether in silico models are credible enough to be used to inform regulation.
Regulatory acceptance
Policymakers are still developing regulations to assess alternatives to animal testing for chemical toxicity. These regulations vary across products and agencies.
For instance, the Organisation for Economic Co-operation and Development, which comprises 38 member countries, has published nearly 100 guidelines on assessing chemical effects on human health and the environment.
The International Cooperation on Alternative Test Methods was created to facilitate chemical toxicity assessment. The many partner organizations within this alliance are making efforts to ensure that alternative methods are scientifically sound, reliable and relevant to human health and environmental safety and that they can be used to replace animal testing in regulatory decision-making.
With clear regulation and global collaboration, alternatives to animal testing can help advance public health, environmental safety and ethical testing practices.
Brad Reisfeld does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Advertisement

Advertisement
Contact Us
If you would like to place dofollow backlinks in our website or paid content reach out to info@qhubonews.com











