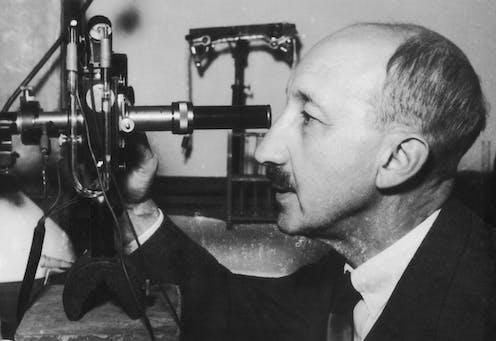
George De Hevesy working in his lab at Stockholm University in 1944. Keystone Features/Hulton Archive via Getty Images
Each October, the Nobel Prizes celebrate a handful of groundbreaking scientific achievements. And while many of the awarded discoveries revolutionize the field of science, some originate in unconventional places. For George de Hevesy, the 1943 Nobel Laureate in chemistry who discovered radioactive tracers, that place was a boarding house cafeteria in Manchester, U.K., in 1911.
Hungarian chemist George de Hevesy.
Magnus Manske
De Hevesey had the sneaking suspicion that the staff of the boarding house cafeteria where he ate at every day was reusing leftovers from the dinner plates – each day’s soup seemed to contain all of the prior day’s ingredients. So he came up with a plan to test his theory.
At the time, de Hevesy was working with radioactive material. He sprinkled a small amount of radioactive material in his leftover meat. A few days later, he took an electroscope with him to the kitchen and measured the radioactivity in the prepared food.
His landlady, who was to blame for the recycled food, exclaimed “this is magic” when de Hevesy showed her his results, but really, it was just the first successful radioactive tracer experiment.
We are a team of chemists and physicists who work at the Facility for Rare Isotope Beams, located at Michigan State University. De Hevesy’s early research in the field has revolutionized the way that modern scientists like us use radioactive material, and it has led to a variety of scientific and medical advances.
The nuisance of lead
A year before conducting his recycled ingredients experiment, Hungary-born de Hevesy had traveled to the U.K. to start work with nuclear scientist Ernest Rutherford, who’d won a Nobel Prize just two years prior.
Rutherford was at the time working with a radioactive substance called radium D, a valuable byproduct of radium because of its long half-life (22 years). However, Rutherford couldn’t use his radium D sample, as it had large amounts of lead mixed in.
When de Hevesy arrived, Rutherford asked him to separate the radium D from the nuisance lead. The nuisance lead was made up of a combination of stable isotopes of lead (Pb). Each isotope had the same number of protons (82 for lead), but a different number of neutrons.
De Hevesy worked on separating the radium D from the natural lead using chemical separation techniques for almost two years, with no success. The reason for his failure was that, unknown to anyone at the time, radium D was actually a different form of lead – namely the radioactive isotope, or radioisotope Pb-210.
Nevertheless, de Hevesy’s failure led to an even bigger discovery. The creative scientist figured out that if he could not separate radium D from natural lead, he could use it as a tracer of lead.
Radioactive isotopes, like Pb-210, are unstable isotopes, which means that over time they will transform into a different element. During this transformation, called radioactive decay, they typically release particles or light, which can be detected as radioactivity.
Through radioactivity, an unstable isotope can turn from one element to another.
This radioactivity acts as a signature indicating the presence of the radioactive isotope. This critical property of radioisotopes allows them to be used as tracers.
Radium D as a tracer
A tracer is a substance that stands out in a crowd of similar material because it has unique qualities that make it easy to track.
For example, if you have a group of kindergartners going on a field trip and one of them is wearing a smartwatch, you can tell if the group went to the playground by tracking the GPS signal on the smartwatch. In de Hevesy’s case, the kindergartners were the lead atoms, the smart watch was radium D, and the GPS signal was the emitted radioactivity.
In the 1910s, the Vienna Institute of Radium Research had a larger collection of radium and its byproducts than any other institution. To continue his experiments with radium D, de Hevesy moved to Vienna in 1912.
He collaborated with Fritz Paneth, who had also attempted the impossible task of separating radium D from lead without success. The two scientists “spiked” samples of different chemical compounds with small amounts of a radioactive tracer. This way they could study chemical processes by tracking the movement of the radioactivity across different chemical reactions
De Hevesy continued his work studying chemical processes using different isotopic markers for many years. He even was the first to introduce nonradioactive tracers. One nonradioactive tracer he studied was a heavier isotope of hydrogen, called deuterium. Deuterium is 10,000 times less abundant than common hydrogen, but is roughly twice as heavy, which makes it easier to separate the two.
De Hevesy and his co-author used deuterium to track water in their bodies. In their investigations, they took turns ingesting samples and measuring the deuterium in their urine to study the elimination of water from the human body.
De Hevesy was awarded the 1943 Nobel Prize in chemistry “for his work on the use of isotopes as tracers in the study of chemical processes.”
Radioactive tracers today
More than a century after de Hevesy’s experiments, many fields now routinely use radioactive tracers, from medicine to materials science and biology.
These tracers can monitor the progression of disease in medical procedures, the uptake of nutrients in plant biology, the age and flow of water in aquifers and the measurement of wear and corrosion of materials, among other applications. Radioisotopes allow researchers to follow the paths of nutrients and drugs in living systems without invasively cutting the tissue.
Radioactive tracers, seen in the top left photo as a white spot and indicated by an arrow in the top right, are often used today in brain scans.
mr. suphachai praserdumrongchai/iStock via Getty Images
In modern research, scientists focus on producing new isotopes and on developing procedures to use radioactive tracers more efficiently. The Facility for Rare Isotope Beams, or FRIB, where the three of us work, has a program dedicated to the production and harvesting of unique radioisotopes. These radioisotopes are then used in medical and other applications.
FRIB produces radioactive beams for its basic science program. In the production process, a large number of unused isotopes are collected in a tank of water, where they can be later isolated and studied.
Scientists Greg Severin and Katharina Domnanich at the Facility for Rare Isotope Beams.
Facility for Rare Isotope Beams.
One recent study involved the isolation of the radioisotope Zn-62 from the irradiated water. This was a challenging task considering there were 100 quadrillion times more water molecules than Zn-62 atoms. Zn-62 is an important radioactive tracer utilized to follow the metabolism of zinc in plants and in nuclear medicine.
Eighty years ago, de Hevesy managed to take a dead-end separation project and turn it into a discovery that created a new scientific field. Radioactive tracers have already changed human lives in so many ways. Nevertheless, scientists are continuing to develop new radioactive tracers and find innovative ways to use them.
Artemis Spyrou receives funding from the National Science Foundation and the Department of Energy.
Sean Liddick receives funding from the Department of Energy and the National Nuclear Security Administration. He is affiliated with the Facility for Rare Isotope Beams.
Katharina Domnanich does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Advertisement

Advertisement
Contact Us
If you would like to place dofollow backlinks in our website or paid content reach out to info@qhubonews.com











