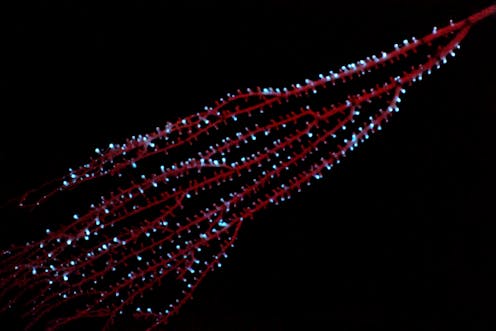
The bamboo coral _Isidella_ displaying bioluminescence in the Caribbean in 2009. Sönke Johnsen, CC BY-ND
Humans have long been fascinated by organisms that can produce light. Aristotle, who was a scientist as well as a philosopher, wrote the first detailed descriptions of what he called “cold light” more than 2,000 years ago. More recently, pioneering researchers like World War II Army veteran Emmett Chappelle and deep submergence vehicle pilot Edith Widder advanced the study of this phenomenon with novel technologies.
At least 94 living organisms produce their own light through a chemical reaction inside their bodies – an ability called bioluminescence. Examples include luminous fireflies, algae that create “glow-in-the-dark” bays, small crustaceans with intricate courtship displays, and deep-sea fish and coral. Yet despite its widespread occurrence, scientists don’t yet know when or where it first emerged, or its original function.
As marine biologists who specialize in deep-sea habitats, we know that bioluminescence is particularly common in the ocean. This indicates that light production may give organisms from across the tree of life a fitness advantage that improves their chances of survival.
Our research focuses on octocorals – soft-bodied corals such as sea fans that have treelike shapes and are found in various places in the world’s oceans. They are a diverse and ancient group of animals that includes some 3,500 species, many of which are bioluminescent.
The colonial false gold coral Savalia displaying bioluminescence in the Caribbean in 2009.
Sönke Johnsen, CC BY-ND
Octocorals can create coral gardens and animal forests in the oceans, particularly in the deep sea. These communities provide homes and nursery habitats for many other animals, including fish and sharks.
All octocorals use the same chemical reaction to bioluminesce. A 2022 study determined the evolutionary relationships among these corals. These genetic connections, and the fact that fossils of octocorals exist, make these animals an ideal focus to investigate when bioluminescence appeared and how it spread across geological time.
Testing for bioluminescence at sea
Over a decade ago, we started testing the ability of different octocoral species to bioluminesce. To produce the glowing light, corals must be stimulated either physically or chemically.
Bioluminescence first piqued our curiosity during a 2014 research cruise on the R/V Celtic Explorer over the Whittard Canyon off the southwest coast of Ireland. We were taking a tissue sample of a bamboo coral collected from the deep seafloor by a remotely operated vehicle.
The vehicle had manipulator arms that allowed the pilot to collect coral specimens and place them in sampling containers to keep the organisms alive and protected as the vehicle surfaced. After this sample came onboard the ship, we used forceps to take a single coral polyp from it in a room with low lighting and saw a flash of blue light.
Since then, we have worked with collaborators from the Monterey Bay Aquarium Research Institute and Tohoku University to record what species are able to glow, either on the ship after collection or as we observe them on the seafloor using low-light cameras. Combined with previous published records, we now know that bioluminescence occurs in approximately 60 coral species. It’s likely that many more await discovery.
For animals that live in dark deep-sea waters, light is an effective way to communicate.
When and why bioluminescence emerged
In a study published in April 2024, we presented the oldest record in geological time for bioluminescence on Earth. We showed that this chemical reaction evolved several millennia earlier than the previous estimate, around the time that life on Earth rapidly diversified over 540 million years ago in a period called the Cambrian Explosion. We determined this by mapping the presence of bioluminescence onto the octocoral tree of life, a graphic tool that biologists use to show evolutionary relationships among species.
Initially, bioluminescence may have evolved to reduce free radicals – chemically unstable atoms that can damage cells. However, at some point, it evolved into a form of communication.
Our results indicate that light signaling was the earliest form of communication in the oceans, and we know that some animals that could detect light evolved during the Cambrian period. Our research indicates that interactions involving light occurred among species during a time when animals were rapidly diversifying and occupying new habitats.
Bioluminescence emerged around the time of the Cambrian Explosion, the most intense burst of evolution on record.
Gaining and losing light
We are continuing to test corals for bioluminescent abilities in a variety of ways. One main component involved in producing light in corals and other animals is an enzyme called luciferase. Using DNA sequence data, we are developing a test for the genetic potential to bioluminesce that will make it easier and less invasive for us to study this trait.
We have preliminary evidence that non-bioluminescent octocorals still have homologous luciferase genes – genetic instructions that were passed down from a common ancestor of all octocorals. Why corals that can’t produce light have retained these genes is a mystery.
Do they produce very low-level light that scientists can’t detect with current methods? Or are their luciferase genes nonfunctional? Further study may show why certain octocorals appear to have lost the ability to bioluminesce, and how this loss may have affected their survival in different habitats.
Our recent results show that many corals that live in shallow waters but arose from deep-water ancestors retained the ability to bioluminesce. It is possible that some corals lost this ability over time as it became less useful in shallower ocean settings with more light.
We also are investigating how bioluminescence has evolved in other creatures, including shrimp that migrate upward from deep waters to feed in the daytime and return to deep waters at night. These animals are exposed to changing light conditions and produce light in multiple, unique ways.
As one notable example, some shrimp vomit light-making chemicals, creating a luminescent spew to fend off predators. They also have external bioluminescent light organs along their body that produce blue light.
Studying creatures like these improves our understanding of how different amounts of light in the environment, including light produced by organisms, affect the evolution of bioluminescence and influence organisms’ vision. This can provide insight into how bioluminescence affected eye evolution and vision some 540 million years ago, when life on Earth was diversifying.
The fact that corals have been able to produce light for hundreds of millions of years implies that this ability has contributed significantly to their survival. Furthermore, our findings support the idea that bioluminescence has been a critical form of communication through geologic time for many types of animals, particularly in the deep sea.
This research has sparked new ideas for us about early animal evolution and communication. Light signaling gave animals a new way to communicate in a rapidly changing time, when new predators and a more complex landscape were emerging. Increased sensory capabilities in the ocean could have been valuable in these conditions. Perhaps bioluminescence is a missing piece of the puzzle that has not yet received full attention in studies of the origin and evolution of animals in deep time.
Andrea Quattrini receives funding from Smithsonian Institution, National Oceanic Atmospheric Administration Office of Ocean Exploration, and the National Science Foundation.
Danielle DeLeo does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Advertisement

Advertisement
Contact Us
If you would like to place dofollow backlinks in our website or paid content reach out to info@qhubonews.com











