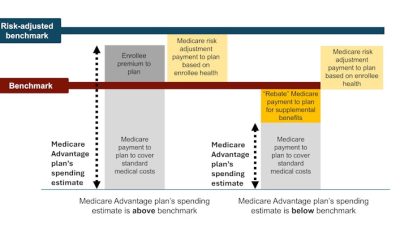
Studying embryogenesis is key to unraveling the mysteries of early life. luismmolina/iStock via Getty Images Plus
Embryonic development, also known as embryogenesis, is a cornerstone in understanding the origins of life. But studying this marvel of intricate and layered biological processes in people faces considerable challenges. Early-stage human embryos are difficult to obtain. Then there are ethical issues surrounding their use. This has made it difficult for scientists to understand early human development.
However, advances in genetic engineering and molecular and cellular biology have catalyzed the emergence of synthetic embryology, a subfield dedicated to replicating and studying embryonic development in a petri dish using human stem cells. By offering new tools to explore the enigmatic earliest stages of human development, synthetic embryology can help researchers overcome the challenges of using real human embryos.
As a reproductive and developmental biologist, I develop stem cell models for embryogenesis. With these new models, researchers can also better understand conditions that affect human reproduction and development as well as maternal-fetal health, potentially leading to new therapies.
Making human embryos from stem cells
Embryogenesis begins with the fertilization of an egg. This triggers the egg to rapidly divide into embryonic cells that soon form an inner cell mass that eventually develops into the fetus and a outer layer of cells that will give rise to the placenta.
Upon implantation in the uterus, the inner cell mass develops into the three layers that will create all the tissues and organs of the human body. Concurrently, the placenta begins to form as the embryo attaches itself to the uterine wall, a crucial step for maternal-fetal connection. This attachment enables the transfer of nutrients, oxygen and waste between mother and fetus.
Synthetic embryology artificially recreates these developmental stages using human pluripotent stem cells derived from human embryos or induced from adult human cells. Like early embryonic cells, these cells have the ability to develop into any type of cell in the human body. In carefully engineered lab environments, researchers can coax these cells to form multicellular structures that mimic various embryonic developmental stages, including early organ formation.
This diagram shows the first few weeks of human embryogenesis, which begins with fertilization.
Jrockley/Wikimedia Commons
Researchers created the first human embryo model from embryonic stem cells in 2014. This pioneering model, also called a gastruloid, captured key aspects of early human development and showed that scientists can drive pluripotent stem cells to form patterned layers echoing the three germ layers and the outer layers of the embryo.
Gastruloids are easy to replicate and measure when studying early events in development. These 2D gastruloids can also help researchers precisely identify and image embryonic cells. However, this model lacks the complex 3D structure and spatial cell interactions seen in natural embryogenesis.
Advancements in human embryo models
Since the first gastruloid, the field has made substantial advancements.
Over the years, various models have been able to replicate different facets of human embryogenesis, such as amniotic sac development, germ layer formation and body plan organization. Researchers have also developed organ-specific models for early organ development, such as a model that captures key events of neural development and fetal lung organoids that mimic the process of lung formation.
However, none of these models fully captures the entire process of a single cell type developing into the complete structure of a whole embryo.
A significant breakthrough occurred in 2021 when several research groups successfully used human pluripotent stem cells with higher developmental potential to create blastoids, which resemble early-stage embryos prior to implantation. Blastoids form in a similar way to human embryos, starting from just a few cells that proliferate and organize themselves.
The developmental and structural similarity of blastoids to embryos make them useful for studying the early steps of how embryos form, especially before they attach to the womb. Blastoids can adhere to lab dishes and undergo further growth. They can also mimic embryo implantation in the uterus by integrating with maternal endometrial cells and developing into later embryonic stages after implantation.
Embryo models allow researchers to study key developmental processes, such as the formation of the spine.
Recently, researchers have successfully created more complex models in the lab that mimic what happens after embryos attach to the womb. Two research teams have used specially engineered cells to create structures similar to those of human embryos at about one week postimplantation. These models are also able to form the cells that eventually turn into sperm and eggs in humans, mirroring what happens in natural development.
Another research group was also able to create a similar model from pluripotent stem cells without needing to genetically engineer them. This model is able to mimic even later development stages and the beginning of nervous system formation.
Choosing the right models
In the evolving field of synthetic embryology, no single model can perfectly capture all aspects of embryogenesis. Consequently, the objective isn’t to play God, creating life in a petri dish, but rather to enhance our understanding of ourselves. This goal underscores the importance of carefully choosing the model best suited to the specific research objectives at hand.
For example, my previous work focused on chromosomal abnormalities in early human development. Aneuploidy, or cells with an abnormal number of chromosomes, is a leading cause of pregnancy loss. But scientific knowledge about how these abnormal cells affect pregnancy and fetal development is very limited.
Since gastruloids can effectively model these aspects of early development, this system could be ideal for studying aneuploidy in early development. It allows researchers to precisely track and analyze how aneuploid cells behave and how they affect developmental processes.
Using this model, my team and I discovered that cells with chromosomal abnormalities are more likely to mature into placental cells and are likely eliminated during the development of fetal cells. This finding offers significant insight into why babies with normal chromosome numbers can be born healthy even with aneuploidy detected during pregnancy. Such discoveries are valuable for improving diagnostic and prognostic methods in prenatal care.
Future models that more completely replicate embryonic structures and more closely mirror biological events will not only advance understanding of the fundamentals of early development but also hold great potential in addressing clinical problems. Researchers can use them to model diseases and develop drugs for early life or genetic conditions. These models are also invaluable for studying tissue formation in regenerative medicine. Creating embryo models from a patient’s own cells could also allow researchers to study the genetics of development and aid in personalizing treatments.
Key to progress in the field of synthetic embryology is unwavering adherence to ethical standards and regulation.
Crucially, these embryo models are neither synthetic nor actual embryos. The International Society for Stem Cell Research strictly prohibits transferring these embryo models into the uterus of a human or an animal. Although these models mimic certain features of early developmental stages, they cannot and will not develop into the equivalent of a human baby after birth. Grounding research in solid justifications and oversight will help ensure that scientific exploration into the fabric of life is conducted with the utmost respect and responsibility.
By embracing the complexities and potential of synthetic embryology, researchers stand on the brink of a new era in biological understanding and are poised to unravel the mysteries of life itself.
Min (Mia) Yang receives funding from University of Washington
Advertisement

Advertisement
Contact Us
If you would like to place dofollow backlinks in our website or paid content reach out to info@qhubonews.com